Advanced Neural Probes
Mapping the brain and peripheral circuits may be the grandest challenge in a all of science today. Our mission is to help neuroscientists, neurologists, and other clinicians understand these amazingly complex circuits with tools that can monitor neural activity or recreate it in a natural or biomimetic way. We've developed expertise in MEMS and microsystem technology because we believe it offers novel ways to combine optical stimulation, recording arrays, drug delivery, biocompatible and flexible materials, and novel packaging solutions.
|
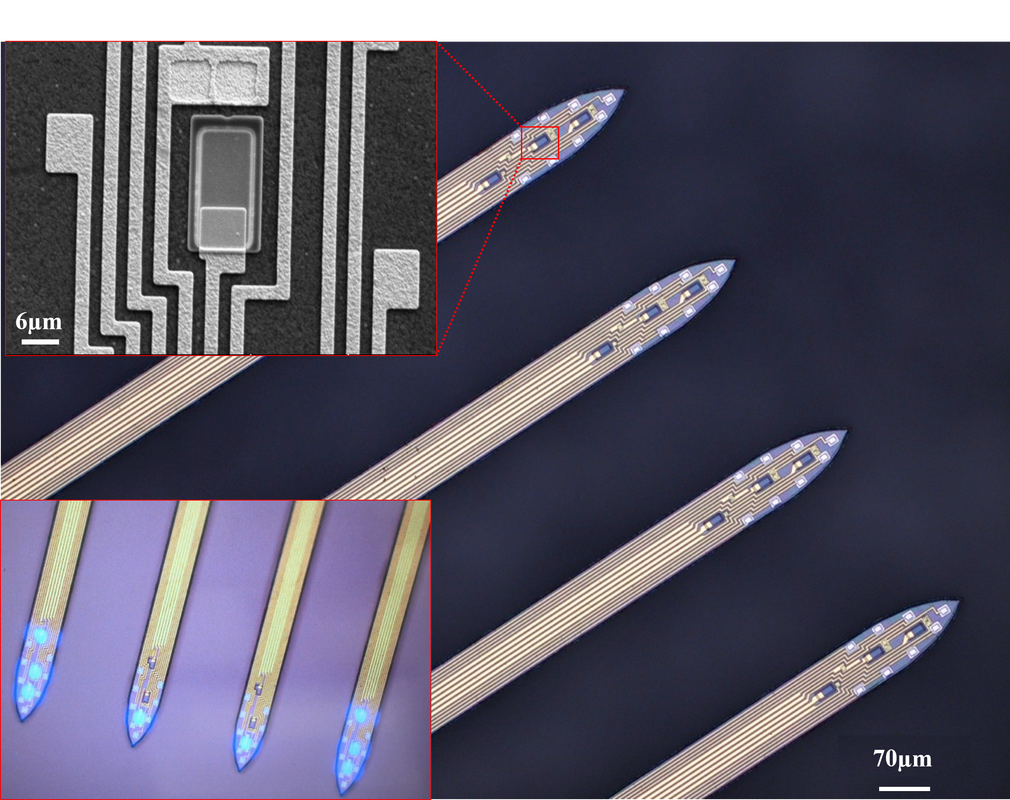
Artifact-free and high-temporal-resolution microLED neural probe for opto-electrophysiology
The combination of in vivo extracellular recording and genetic-engineering-assisted optical stimulation is a powerful tool for the study of neuronal circuits. Precise analysis of complex neural circuits requires high-density integration of multiple cellular-size light sources and recording electrodes. Our lab developed the minimal-stimulation-artifact (miniSTAR) μLED optoelectrodes that enable effective elimination of stimulation artifact. A multi-metal-layer structure with a shielding layer effectively suppresses capacitive coupling of stimulation signals. With a heavily boron-doped silicon substrate combined with transient stimulation pulse shaping, we reduced stimulation artifact on miniSTAR μLED optoelectrodes to much smaller than a typical spike detection threshold. MiniSTAR μLED optoelectrodes will facilitate functional mapping of local circuits and discoveries in the brain. [Kim, Kanghwan, et al. "Artifact-free and high-temporal-resolution in vivo opto-electrophysiology with microLED optoelectrodes." Nature Communications, 11:2063, May 2020.] [Link] |
|
|
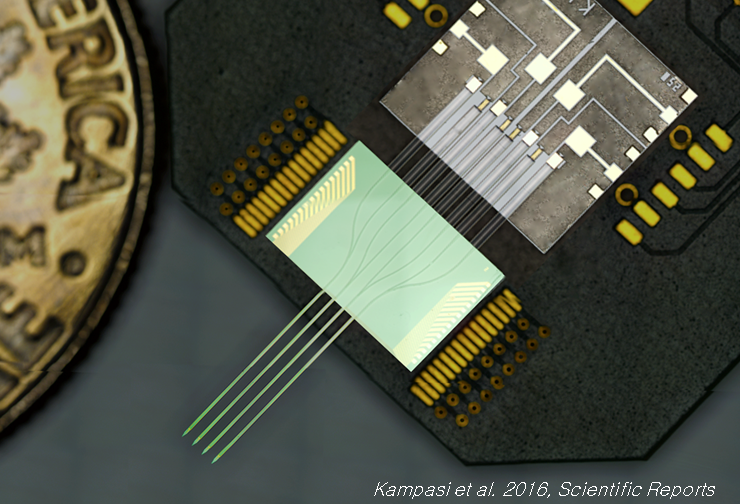
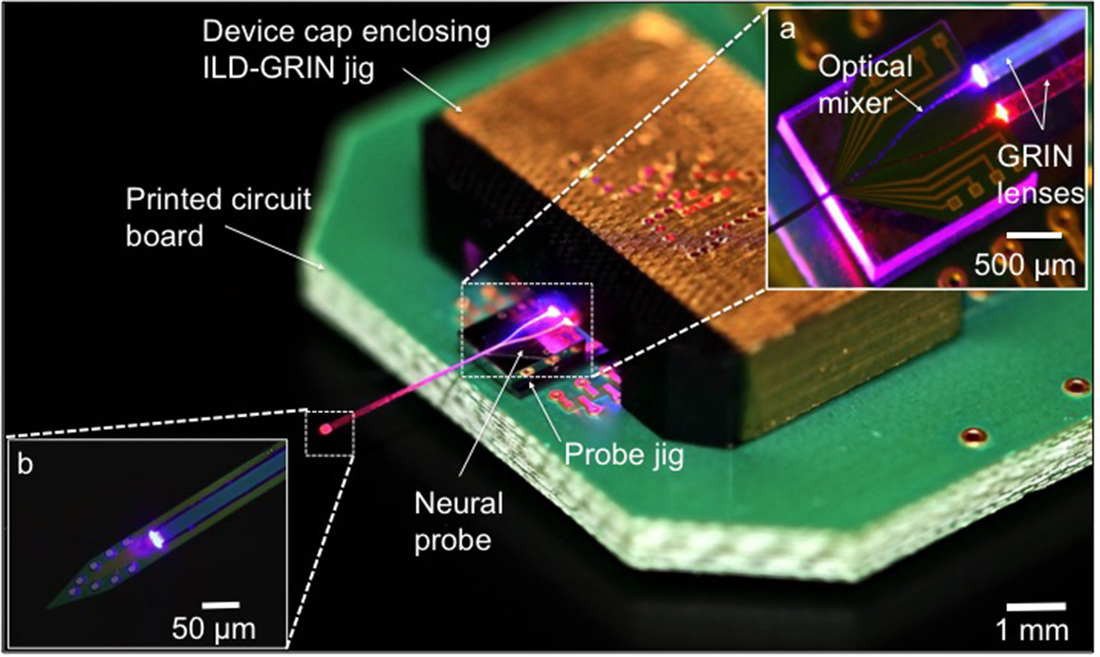
Dual color optogenetic control of neural populations using low-noise, multishank optoelectrodes
We report a scalable multisite optoelectrode design that allows simultaneous optogenetic control of two spatially intermingled neuronal populations in vivo. We describe the design, fabrication, and assembly of low-noise, multisite/multicolor optoelectrodes. Each shank of the four-shank assembly is monolithically integrated with 8 recording sites and a dual-color waveguide mixer with a 7 × 30 μm cross-section, coupled to 405 nm and 635 nm injection laser diodes (ILDs) via gradient-index (GRIN) lenses to meet optical and thermal design requirements. To better understand noise on the recording channels generated during diode-based activation, we developed a lumped-circuit modeling approach for EMI coupling mechanisms and used it to limit artifacts to amplitudes under 100 μV upto an optical output power of 450 μW. We implanted the packaged devices into the CA1 pyramidal layer of awake mice, expressing Channelrhodopsin-2 in pyramidal cells and ChrimsonR in paravalbumin-expressing interneurons, and achieved optical excitation of each cell type using sub-mW illumination. We highlight the potential use of this technology for functional dissection of neural circuits. [K. Kampasi, D. F. English, J. Seymour, E. Stark, S. McKenzie, M. Vöröslakos, G. Buzsáki, K. D. Wise and E. Yoon, “Dual Color Optogenetic Control of Neural Populations Using Low-Noise, Multishank Optoelectrodes,” Microsystems and Nanoengineering, 4, 10, June 2018.] [Link] |
|
|
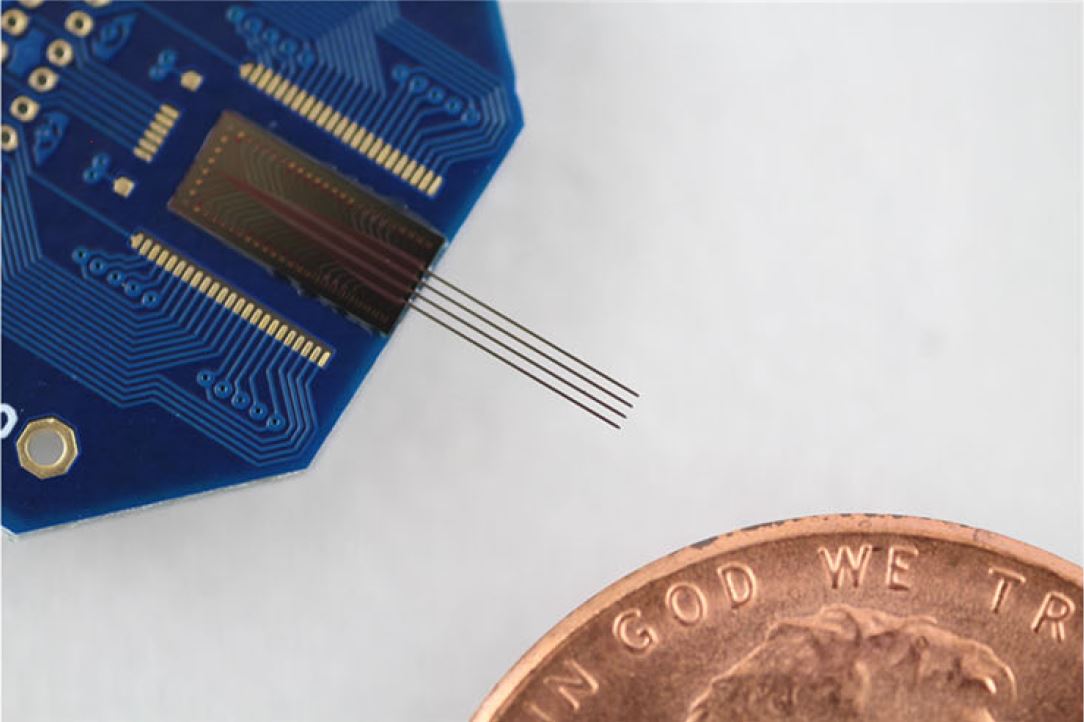
Multicolor optoelectrode for bidirectional control of local neural circuits
Maximizing the potential of optogenetic approaches in deep brain structures of intact animals requires optical manipulation of neurons at high spatial and temporal resolutions, while simultaneously recording electrical data from those neurons. Here, we present the first fiber-less optoelectrode with a monolithically integrated optical waveguide mixer that can deliver multicolor light at a common waveguide port to achieve multicolor modulation of the same neuronal population in vivo. We demonstrate successful device implementation by achieving efficient coupling between a side-emitting injection laser diode (ILD) and a dielectric optical waveguide mixer via a gradient-index (GRIN) lens. The use of GRIN lenses attains several design features, including high optical coupling and thermal isolation between ILDs and waveguides. We validated the packaged devices in the intact brain of anesthetized mice co-expressing Channelrhodopsin-2 and Archaerhodopsin in pyramidal cells in the hippocampal CA1 region, achieving high quality recording, activation and silencing of the exact same neurons in a given local region. This fully-integrated approach demonstrates the spatial precision and scalability needed to enable independent activation and silencing of the same or different groups of neurons in dense brain regions while simultaneously recording from them, thus considerably advancing the capabilities of currently available optogenetic toolsets. [Kampasi K., Stark E., Seymour J., Na K., Winful H. G., Buzsaki G., Wise K. D., Yoon E., "Fiberless multicolor neural optoelectrode for in vivo circuit analysis" Scientific Reports, 6, 30961, 2016.] [Link] |
|
|
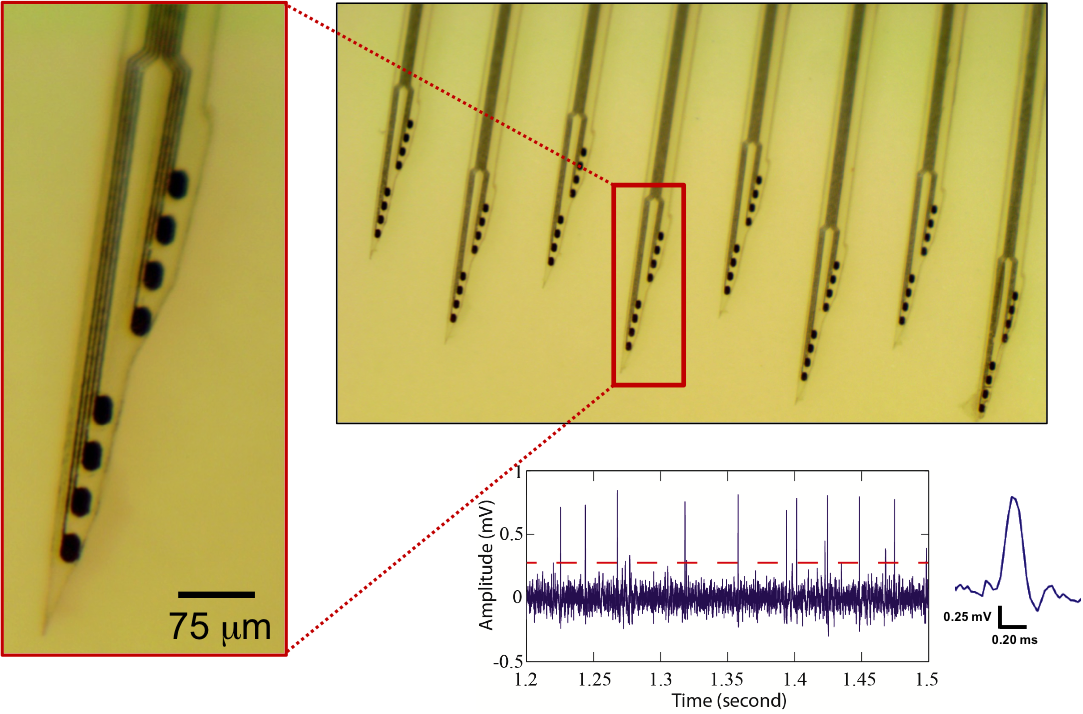
Neural Probe with Monolithically Integrated micro-LEDs
In this project, we designed and demonstrated an innovative solution to enhance both spatial resolution and scalability of optogenetic stimulation and recording probes. We monolithically integrated cell-sized LEDs in microns in dimensions (micro-LEDs) and recording electrodes on silicon probe shanks, with all dimensions defined with a resolution of < 1 mm. We demonstrate that these "micro-LED probes" enable control of spiking of single neurons and induce field oscillations of neuronal activity in the intact brain of freely moving mice with unprecedented resolution, so that optical stimulation of a very specific neural circuit is no longer limited by the light delivery methodology, but rather is rather bottlenecked by the expression specificity of current opsin technologies. [F. Wu, E. Stark, P.-C. Ku, K. D. Wise, G. Buzsaki, E. Yoon, “Monolithically Integrated mLEDs on Silicon Neural Probes for High-Resolution Optogenetic Studies in Behaving Animals,” Neuron, 88, pp. 1136-1148, doi: 10.1016, 2015.] [Link] |
|
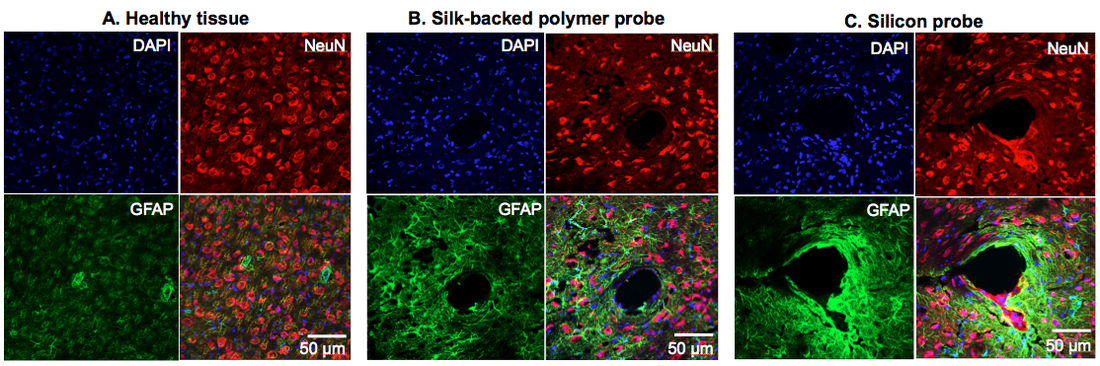
Silk-coated Polymer Neural Probes for Chronic Recording
Chronic neural recording has long been challenged by the inevitable immune response that degrades the implanted electrode performance over time. This project presents a polymer probe with a unique geometry designed to minimize the tissue encapsulation near the electrode sites. By utilizing silk as a stiff and biodegradable coating, we obtain the necessary strength for insertion of the flexible and miniaturized probe into the brain tissue. [F. Wu, L. W. Tien, F. Chen, J. D. Berke, D. L. Kaplan, E. Yoon, “Silk-backed structural optimization of high-density flexible intracortical neural probes,” IEEE J. Microelectromechanical Syst., vol. 24, no.1, pp. 62-69, 2015.] Histology findings: Preliminary histology result showing 12-week post-implantation immunoreactivity response: (A) healthy cortical tissue; (B) silk-backed parylene probe; (C) silicon probe. These representative 40X confocal immuno-fluorescent images from the same tissue section qualitatively depict a more reactive tissue response (GFAP (green), NeUN (red) and DAPI (blue)) around the silicon probe as compared to the silk-backed parylene probe.
[K. Kampasi, B. Gross, J. Seymour, F. Wu, L. W. Tien, D. L. Kaplan, E. Yoon, G. Poe, "Biocompatibility of Dissolvable Silk Fibroin in Central Nervous System,” BMES-MBECC, Nov. 2014] |
|
|
Neural Probe With Monolithically Integrated Waveguide
To complement the rapid advancement in optogenetic neuroscience, we have developed MEMS neural probes with integrated optical waveguides and recording electrodes to simultaneously stimulate targeted neurons with light and record the induced electrical neural activities. Video: Acute rat experiment at the Buzsaki Lab (Rutgers University) using the waveguide neural probe. Sinusoidal light input (0-100 Hz) induces spiking activities from multiple cells. Bottom trace (black) shows the light waveform. Blue and red ticks show the spike timing of interneurons and pyramidal cells, respectively, which are phase-locked precisely to the peak of the sine wave . Each horizontal series of ticks represent an isolated cell. This video demonstrates the probe's capability to manipulate and understand brain functions at the cellular level. [F. Wu, E. Stark, M. Im, I-J. Cho, E-S. Yoon, G. Buzsáki, K. D. Wise, E. Yoon, “An Implantable Neural Probe with Monolithically Integrated Dielectric Waveguide and Recording Electrodes for Optogenetics Applications,” IEEE J. Neural Eng., vol. 10, 056012, 2013.] [M. Im, I.-J. Cho, F. Wu, K. D. Wise, and E. Yoon, “Neural probes integrated with optical mixer/splitter waveguides and multiple stimulation sites,” in Proc. MEMS 2011 Conference, Cancun, Mexico, January 23-27, 2011, pp. 1051-1054.] |
Other Biointerface Research
|
|
Artificial Smart Skin 1: Dual-Mode Tactile Sensor
We have implemented a dual mode proximity sensor which can detect not only proximity but also touch of an object for robot assistant applications. The sensor operates in two modes: proximity mode and tactile mode. Initially, the sensor operates in proximity mode until it touches an object. When the proximity mode detects the contact of any objects, the sensor will switch its mode into tactile sensing mode for acquiring an image from an object. [H.-K. Lee, S.-I. Chang, and E. Yoon, “Dual-Mode Capacitive Proximity Sensor for Robot Application: Implementation of Tactile and Proximity Sensing Capability on a Single Polymer Platform Using Shared Electrodes,” IEEE Sensors Journal, Vol. 9, No. 12, pp. 1748-1755, Dec. 2009] [H.-K. Lee, S.-I. Chang, and E. Yoon, “A Flexible Polymer Tactile Sensor: Fabrication and Modular Expandability for Large Area Deployment,” IEEE J. Microelectromech. Sys., vol. 5, no. 6, pp. 1681-1686, 2006.] |
|
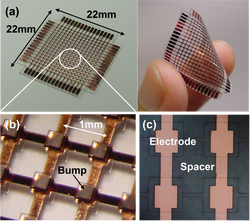
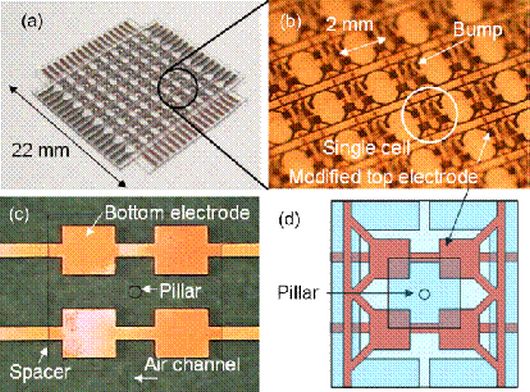
Artificial Smart Skin 2: Normal and Shear Force Measurement
We have developed a new flexible capacitive tactile sensor array with the capability of measuring both normal and shear force distribution using polydimethylsiloxane (PDMS) as a base material. A tactile cell consists of two thick PDMS layers with embedded electrodes, an air gap, and a pillar structure. The pillar structure is formed at the center of each tactile cell between the air gap under a large bump. There are four capacitors in a cell to decompose the contact force into normal and shear components. [H.-K. Lee, J. Chung, S.-I. Chang, and E. Yoon, “Normal and Shear Force Measurement Using a Flexible Polymer Tactile Sensor With Embedded Multiple Capacitors,” IEEE J. Microelectromech. Sys., Vol. 17, No. 4, pp. 934-942, Aug., 2008.] |